Summary
You have heard about the Transwell Migration Assay and the Invasion Assay, but what exactly are they, and importantly, what is the difference? How are they used, what do they tell you about your cells in culture, and how are they best quantified? This blog post will give you the complete breakdown of the assays and answer all of your questions.
What is the Transwell Migration Assay?
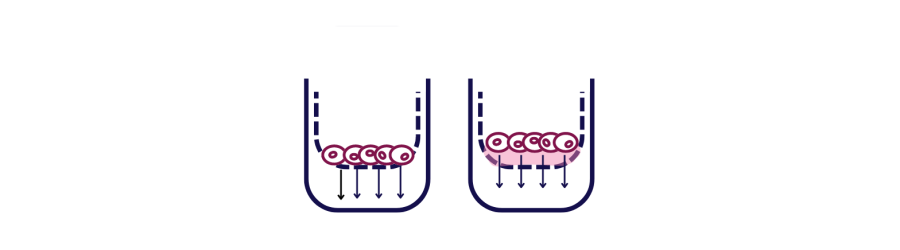
In a typical migration assay, cells are placed in the upper chamber of a Transwell insert and allowed to migrate through a porous membrane towards a chemoattractant in the lower chamber. The extent of cell movement is then measured, providing insights into the factors that influence cell motility.
The Transwell migration assay (‘migration assay hereafter) is simpler than an invasion assay, focusing solely on the cells’ ability to move in response to various stimuli. The assay helps researchers to investigate how cells respond to different chemical signals (chemotaxis), evaluate the effects of drugs or treatments on cell movement, and explore the mechanisms underlying disease processes, including cancer metastasis. By analyzing cell migration, researchers can gain valuable information about cellular dynamics and their implications in health and disease.
The cell migration assay protocol in brief:
- Cells are placed in the upper chamber of a Transwell insert.
- The lower chamber contains a chemoattractant (a substance that attracts cells).
- Cells migrate through a porous membrane towards the chemoattractant.
- The number of cells that have moved to the lower chamber is quantified.
What is the Transwell Invasion Assay?
The invasion assay works by:
- A layer of Matrigel (a gelatinous protein mixture resembling the ECM) is applied on the porous membrane of a Transwell insert.
- Cells are placed in the upper chamber, on top of the Matrigel layer.
- The lower chamber contains a chemoattractant.
- Cells must degrade the Matrigel and migrate through it to reach the porous membrane and move towards the chemoattractant in the lower chamber.
- The number of cells that have invaded through the Matrigel and membrane is quantified.
Access our webinar Automating Transwell Migration/ Invasion Assay with AI-Based Image Analysis to learn more.
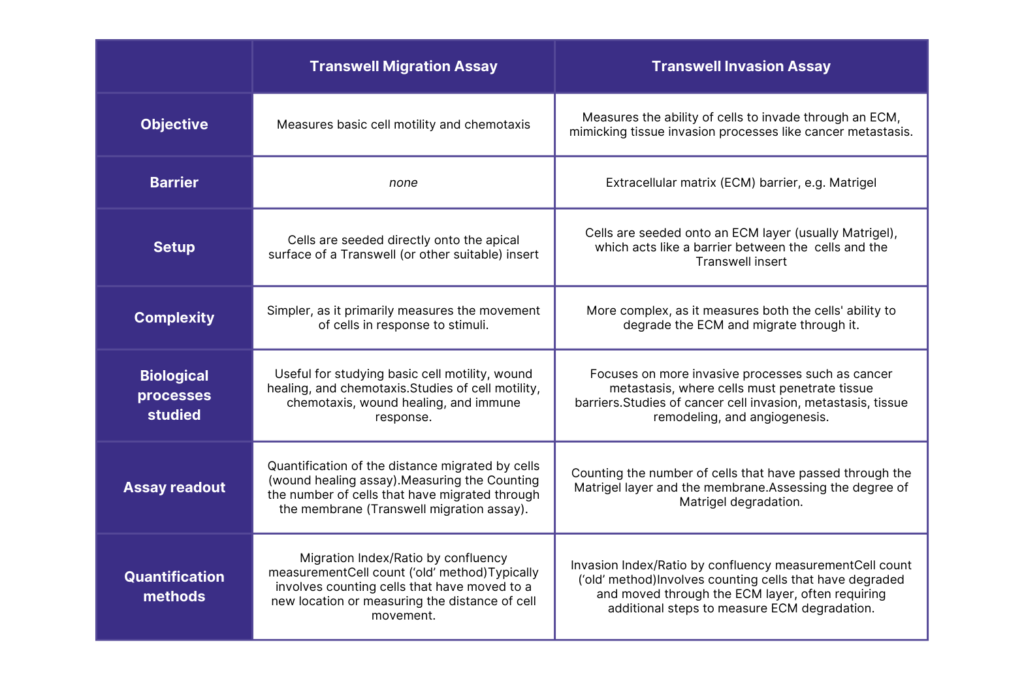
What is the difference between the Migration and Invasion Assays?
Differences Between Migration and Invasion Assays
Data read-out from the Transwell Invasion Assay
From a Transwell invasion assay, you typically obtain quantitative and qualitative data related to the amount and behavior of cells that have successfully invaded through the extracellular matrix (ECM) barrier and migrated to the other side of the membrane.
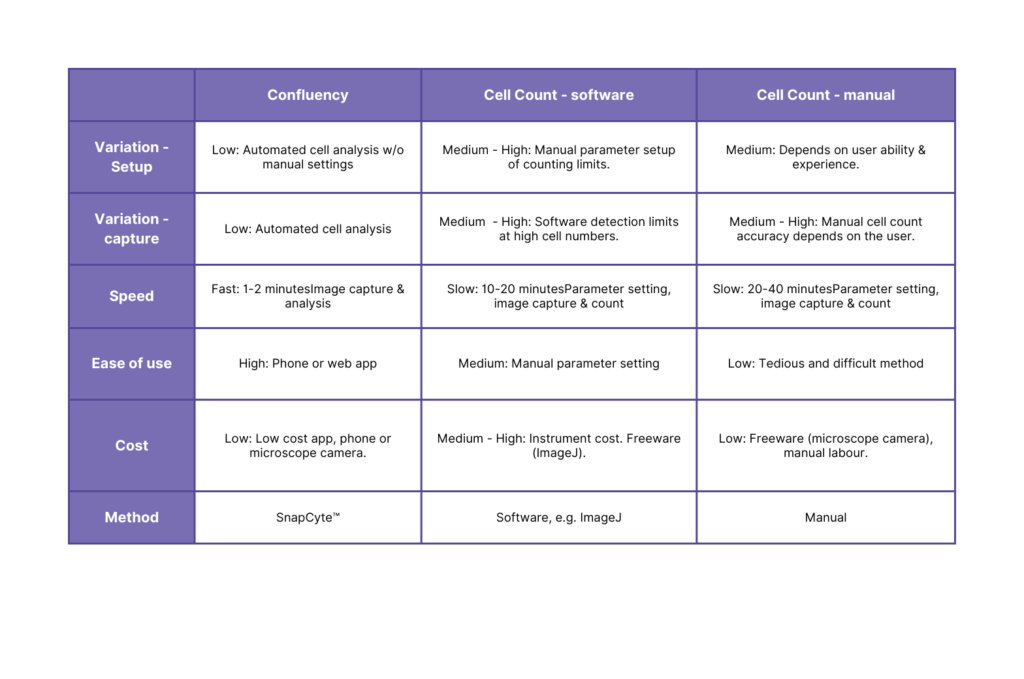
Calculating the Invasion Ratio
- Detect degree of invasion by cell confluency
- Detect degree of invasion by cell count
-
- Manual variation: In either manual counting or image analysis, setting the parameters for which to count are done manually, and thus are subject to bias by the user.
- Time-consuming: Whether you are to set up the software criteria or count manually, it takes a LOT of time to conduct this method!
- Absorbance or Fluorescence Intensity:
Morphological observations
Qualitative data on cell morphology can be gathered, including cell size, shape, nuclear morphology, cell-matrix interactions, invasion depth, and cytoskeletal organization. Many of these morphological characteristics require further analysis methods, such as immune-staining of cells and ECM proteins and high resolutions imaging.
Morphological data provide valuable information on how cells physically interact with the ECM, the structural adaptations they undergo during invasion, and the mechanisms driving their invasive behavior. Analyzing these features helps in understanding the cellular processes involved in tissue invasion, particularly in the context of cancer metastasis.
Quantifying Matrigel degradation in the Invasion Assay
The degradation of Matrigel during an invasion assay can be analyzed using several methods that focus on assessing the breakdown of the extracellular matrix (ECM) components, including including immunohistochemistry (IHC), immunofluorescence, Enzyme-Linked Immunosorbent Assay (ELISA), and directly by microscopy and image analysis. These methods help to understand the invasive potential of cells and the role of proteolytic enzymes like matrix metalloproteinases (MMPs) in this process.
Method comparison: Migration/Invasion Ratio quantification
If you want to learn more about analyzing the invasion assay using SnapCyte™, see this Case Study: Invasion assay analysis in less than 30 minutes using AI.
What is the Transwell vs. the Boyden chamber?
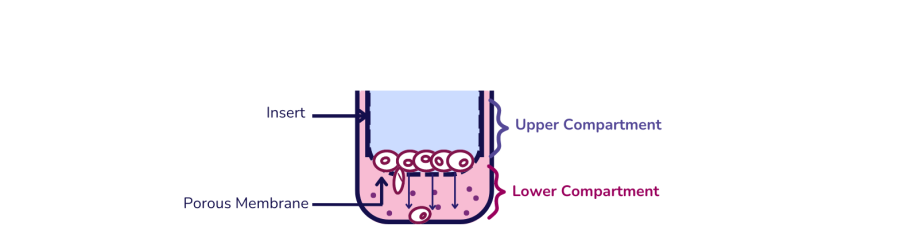
The Transwell and Boyden chambers are both devices used in cell migration and invasion assays, though they are often described in different contexts.
The Transwell chamber features a cell culture insert with a porous membrane separating two compartments: the upper and lower chambers. This design allows researchers to assess cell migration by placing cells in the upper chamber and adding a chemoattractant to the lower chamber. Cells move through the membrane towards the chemoattractant. For invasion assays, the membrane is coated with an extracellular matrix (ECM) component like Matrigel, requiring cells to degrade the ECM before migrating.
The Boyden chamber is historically referred to in earlier research contexts, where the assay is also often called a transmigration assay. It has a similar design with a porous membrane dividing two compartments. The key difference is that the Boyden chamber is considered a precursor to the modern Transwell system, which is now more widely used and standardized in research.
In essence, while both chambers serve similar purposes in studying cell movement and invasive behavior, the Transwell chamber is the contemporary and commonly used device, whereas the Boyden chamber represents an earlier version of the same basic concept.
Can I use the invasion assay to measure diapedesis?
Key Considerations:
- ECM Layer: In the context of diapedesis, the extracellular matrix (ECM) coating used in a typical invasion assay should be modified to better mimic the endothelial layer that immune cells naturally cross. This involves using endothelial cells and/or specific ECM components that closely resemble the blood vessel environment.
- Endothelial Cells: For a more accurate model of diapedesis, culture endothelial cells on the membrane of the Transwell insert to create a barrier that immune cells must traverse, simulating the process of crossing blood vessel walls.
- Chemotactic Gradients: To effectively study diapedesis, ensure that the appropriate chemotactic gradients (e.g., chemokines) are established to mimic the signals that guide immune cells during the diapedesis process.
Want to know even more about the Invasion Assay?
We hope this blog post explains all that you need to know about the Transwell Migration and Invasion Assays. If not, send us your question and let’s have a conversation about your protocol and assay needs.
You can also:
- Access our webinar Automating Transwell Migration/ Invasion Assay with AI-Based Image Analysis.
- See this Case Study: Invasion assay analysis in less than 30 minutes using AI.
References
- AJ Ridley et al: Cell Migration: integrating signals from front to back. Science 302, 1704-1709. 10.1126/science.1092053
- K Hulkower and R Herber: Cell migration and invasion assays as tools for drug discovery. 2011 Mar; 3(1): 107–124. doi: 10.3390/pharmaceutics3010107
- HM Stoellinger and AR Alexanian: Modifications to the Transwell Migration/Invasion Assay Method That Eases Assay Performance and Improves the Accuracy. Assay Drug Dev Technol. February/March 2022; 20(2): 75–82. doi: 10.1089/adt.2021.140
- CR Justus et al: In vitro Cell Migration and Invasion Assays. J Vis Exp. 2014; (88): 51046. doi: 10.3791/51046